To carry out a myriad of capabilities inside our physique’s cells, many kinds of hard-working protein slip into and out of dense droplets referred to as condensates to speed up biochemical reactions as wanted.
However this fluidity can crumble in illnesses – similar to Alzheimer’s illness, Parkinson’s, and amyotrophic lateral sclerosis (ALS) – that are marked by stable aggregates of lumpy proteins that kind in nerve cells.
Now, a staff of researchers has developed a brand new strategy for imaging the second when proteins recognized to combination in neurodegenerative illnesses start to mass collectively.
Forming hallmark constructions like clumps, plaques, and tangled fibrils, “the proteins now not exhibit speedy reversibility again to liquid kind,” explains protein biophysicist Yi Shen, of the College of Sydney, who led the research.
“It’s subsequently essential to watch condensate dynamics, as they immediately have an effect on pathological states.”
Previous analysis has proven proteins that combination in ALS, a debilitating illness affecting motor operate, exist in a ‘supersaturated’ state at very excessive concentrations that exceed their typical solubilities. In different phrases, these proteins are teetering on the sting as soluble varieties, and are vulnerable to solidifying if the cell turns into overwhelmed.
To take a more in-depth take a look at the behaviors of proteins like these, Shen and colleagues developed two new methods of carefully monitoring the transition of a protein from its liquid to stable section.
Their first take a look at was for a DNA/RNA binding molecule referred to as fused in sarcoma (FUS) protein, which aggregates in ALS and frontotemporal dementia.
When proteins like FUS focus as a gel in condensates, a dense, protein-rich section is surrounded by a dilute one depleted of molecules. Bringing so many proteins shut collectively can tip the combination in the direction of aggregating even additional, irreversibly into stable clumps.
The researchers imaged options of FUS condensates as they fashioned over 24 hours, utilizing two approaches which collected the sunshine refracted via, and scattered again from, the dense protein ‘balls’. The patterns mirrored the interior constructions and density of the condensates, which solidified after 5 days.
“We use a quick digicam to document lengthy vivid area sequences of photos at a excessive body charge. This permits us to discover concurrently quick (buying at excessive body charges) and sluggish dynamics (by buying for a very long time),” Shen and colleagues write of their revealed paper.
Like an explosion within the evening, you’ll be able to see the fluorescent inexperienced proteins emerge out of the blackness as they combination collectively, showing to drag increasingly more proteins out of answer on the condensates’ edges till the entire mass seemingly implodes.
frameborder=”0″ permit=”accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture; web-share” allowfullscreen>
In the event you missed that within the time-lapse video above, watch carefully once more: it reveals the transition from liquid to stable protein begins on the outer fringe of the spherical condensate, which thickens and migrates inwards to the core till the entire droplet turns into a solid-like gel.
“This can be a large step ahead to understanding how neurogenerative illnesses develop from a elementary perspective,” says Shen.
“We are able to now immediately observe the transition of those essential proteins from liquid to stable on the nanoscale – a millionth of a metre in scale,” provides Daniele Vigolo, a biomedical engineer additionally on the College of Sydney.
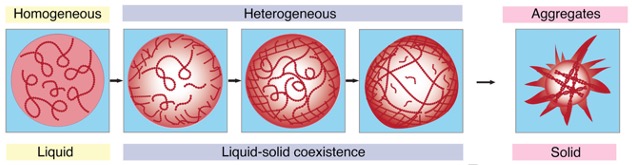
Whereas the experiments had been performed in options of lab-made proteins, and there is clearly an entire lot extra happening inside cells, the researchers say their findings present new insights into the elemental, bodily course of underlying neurodegenerative illnesses.
If the imaging strategies may be replicated with different proteins, we would simply be taught an entire lot extra concerning the unusual methods these proteins work together.
The research has been revealed in PNAS.


