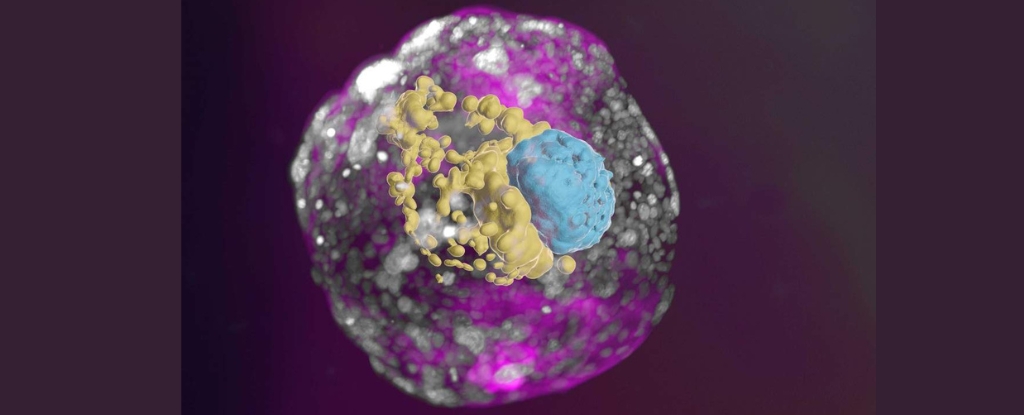
Scientists have developed human embryo fashions from stem cells cultured in a lab, that offers an unprecedented view of the important first week after implantation into the uterine wall.
Fertility, early being pregnant loss, and developmental beginning defects can all be higher understood with a deeper dive into what occurs instantly after a fertilized egg burrows into the womb’s lining at the beginning of gestation. However moral and technical challenges have hampered our means to review these important phases of human embryo growth.
“The drama is within the first month, the remaining eight months of being pregnant are primarily a lot of development,” says molecular geneticist Jacob Hanna from the Weizmann Institute of Science in Israel.
“However that first month remains to be largely a black field. Our stem cell–derived human embryo mannequin provides an moral and accessible manner of peering into this field.”
The worldwide analysis workforce coaxed genetically unmodified, undifferentiated human-derived stem cells into advanced constructions that mimic human embryonic growth.
The method reveals the exceptional self-organizing means of human stem cells, increasing on a latest breakthrough within the technology of embryonic-like stem cells to offer researchers with a brand new method to examine phenomena which have till now been obscured by sensible and moral challenges.
Key options not seen in earlier fashions embody the three lineages that make the placenta and embryonic assist constructions, in addition to the layer of cells that kind an embryo earlier than it folds in on itself and grows into completely different tissues and organs.
Earlier analysis confirmed that stem cells faraway from mouse embryos can nonetheless be artificially guided to develop into tissues that assist and make up the embryo itself, self-assembling right into a structural stem-cell primarily based embryo mannequin (SEM) on the post-gastrulation stage the place the embryonic cells kind into the three essential forms of physique tissue.
“Right here, we lengthen these findings to people, whereas utilizing solely genetically unmodified human naïve embryonic stem cells,” writes Hanna and colleagues.
“We proceeded to check the capability to kind embryo-like constructions… that might mimic completely different phases of pure human in utero growth.”
frameborder=”0″ permit=”accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture; web-share” allowfullscreen>
They decided optimum situations together with cell numbers, ratios inside cell mixtures, and tradition compositions for varied phases, ranging from when implantation happens 7–8 days after fertilization.
“These human full SEMs demonstrated developmental development dynamics that resemble key hallmarks of post-implantation stage embryogenesis as much as 13-14 days post-fertilization,” the authors write.
The fashions depict the meeting of all of the identified lineages and elements of early-stage human embryos, together with the epiblast, hypoblast, extraembryonic mesoderm, trophoblast, and the yolk sac.
frameborder=”0″ permit=”accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture; web-share” allowfullscreen>
Cell profiles from the human SEMs dataset had been discovered to resemble the gene expression patterns and cell kind composition in human embryos shortly after implantation, when in comparison with a reference dataset.
Human SEMs usually are not an identical to embryos, the authors observe, however they’re a mannequin that opens up enormous analysis potentialities.
“Many failures of being pregnant happen within the first few weeks, typically earlier than the lady even is aware of she’s pregnant. That is additionally when many beginning defects originate, although they are usually found a lot later,” Hanna says.
“Our fashions can be utilized to disclose the biochemical and mechanical indicators that guarantee correct growth at this early stage, and the methods wherein that growth can go incorrect.”
The examine has been printed in Nature.